Accelerate Your EU MDR Audit Readiness
Navigating the EU Medical Device Regulation (MDR) can be complex – especially when it comes to Quality Management Systems (QMS) compliance. BSI’s EU MDR QMS Internal Auditor and Lead Auditor Practitioner qualifications provide the knowledge, practical skills, and confidence to ensure effective audits aligned with ISO 13485 and EU MDR expectations.
Choose the Right Path for Your Role:
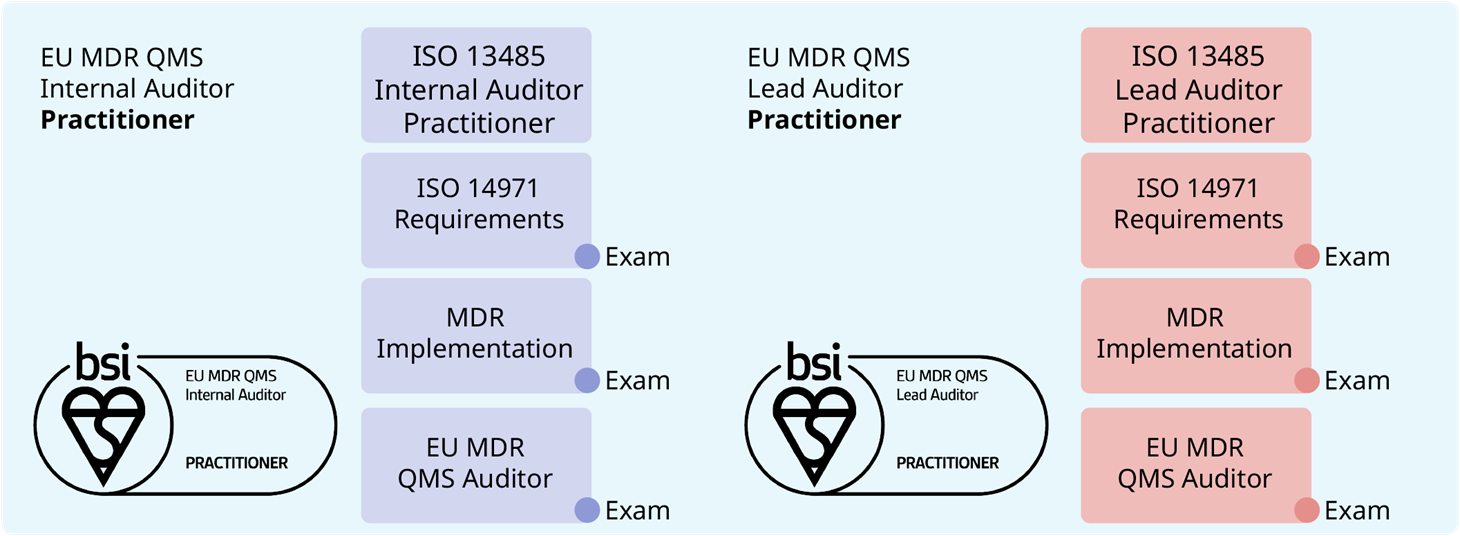
EU MDR QMS Internal Auditor Practitioner (9 Days)
Perfect for those involved in conducting internal audits within an organization's medical device QMS.
- Understand how to plan and execute EU MDR-focused internal audits
- Learn how to assess conformity and report findings effectively
- Gain practitioner-level recognition to support your professional development
EU MDR QMS Lead Auditor Practitioner (12 Days)
Ideal for professionals who need to lead, manage, or guide a team through QMS audits to EU MDR requirements.
- Deepen your knowledge of ISO 13485 and EU MDR audit principles
- Learn how to lead an audit team and manage audit programmes
- Achieve formal recognition as a Lead Auditor Practitioner
What's included in the Packages:
Comprehensive and designed for professionals leading audit programmes or preparing for notified body audits. You will gain technical, regulatory, and leadership expertise through:
- ISO 13485 Internal (2 days) / Lead Auditor (5 days) Practitioner (+ 4 hours on-demand eLearning)
- ISO 14971:2019 Risk Management for Medical Devices (0.5 day eLearning)
- Implementation of MDR 2017/745 for CE Marking (3 days)
- EU MDR QMS Auditor Training (3 days)
Who should attend
- RA, QM, and QA professionals who already perform audits,
- Anyone concerned with certification or active in projects for CE-marking, especially involved in the QMS implementation side
- Staff involved in audits and working for organizations that partner with Medical Device manufacturers e.g. as subcontractor, crucial supplier, OEM, Authorized representative, importer, distributor, auditee
Ready to Build Your EU MDR Audit Capability?
Contact us to discuss which pathway suits your experience and goals best.
Phone: +65 6270 0777
Email: info.sg@bsigroup.com