Please complete the form and click Download to receive the materials explained below.
MDR Documentation Submissions: Best Practices Guidelines
The two most frequent reasons for delays to technical documentation reviews are first: BSI has not been provided with all of the information needed for the review, and second: the information is present within the technical documentation, but is difficult to locate. To reduce the frequency of the above issues, BSI Medical Devices Group proposes this guidance, informally known as “MDR Documentation Submissions: Best Practices Guideline."
Quality Management System (QMS) Aspects of the EU MDR and IVDR
This webinar details the quality management system (QMS) aspects for the new EU Medical Device Regulation (MDR) and EU In Vitro Device Regulation (IVDR) including immediate checks required as well as those for post market introduction. It also covers transition from the current EU Medical Device Directive (MDD) to MDR as well as what BSI as a notified body will require in the QMS portion of their audit and how it interacts with ISO 13485, ISO 9001, MDD, AIMD and IVDD requirements.
Medical Devices Regulation(MDR) Mapping Guide
A guide to help you to map the MDR Safety and Performance Requirements (SPRs) to the Essential Requirements for Medical Device Directive (MDD), Active Implantable Medical Device Directive (AIMD). The document also lists other relevant information which can help you in planning your transition to the MDR.
General safety & performance requirements of the MDR
The webinar covers Annex I/Chapter I: General SPR Requirements including performance, lifetime support, risk management and non-medical devices; Annex II/Chapter II: Packaging/sterility, medicinals/biologicals, chemical/physical/biological properties and device-specific requirements; Annex III Chapter III: Device-supplied information including labeling and IFU.
EU 2017/745 MDR Frequently asked questions
Answers questions such as what happens if a certificate isn't issued before transition period ends? Will new requirements be enforced retrospectively? How MDR will be implemented? What is the impact of the MDR on existing quality management systems? And much more.
MDR Article 18: Implant card requirements and information to be supplied to implant patients
This webinar covers the EU 2017/245 MDR Article 18 implanted medical device (i.e. cardio defibrillator/pacemakers, artificial joints/hardware, breast implants, etc.) issued patient card requirements including background, regulation text, Notified Body assessments, implant card examples and frequently asked questions.
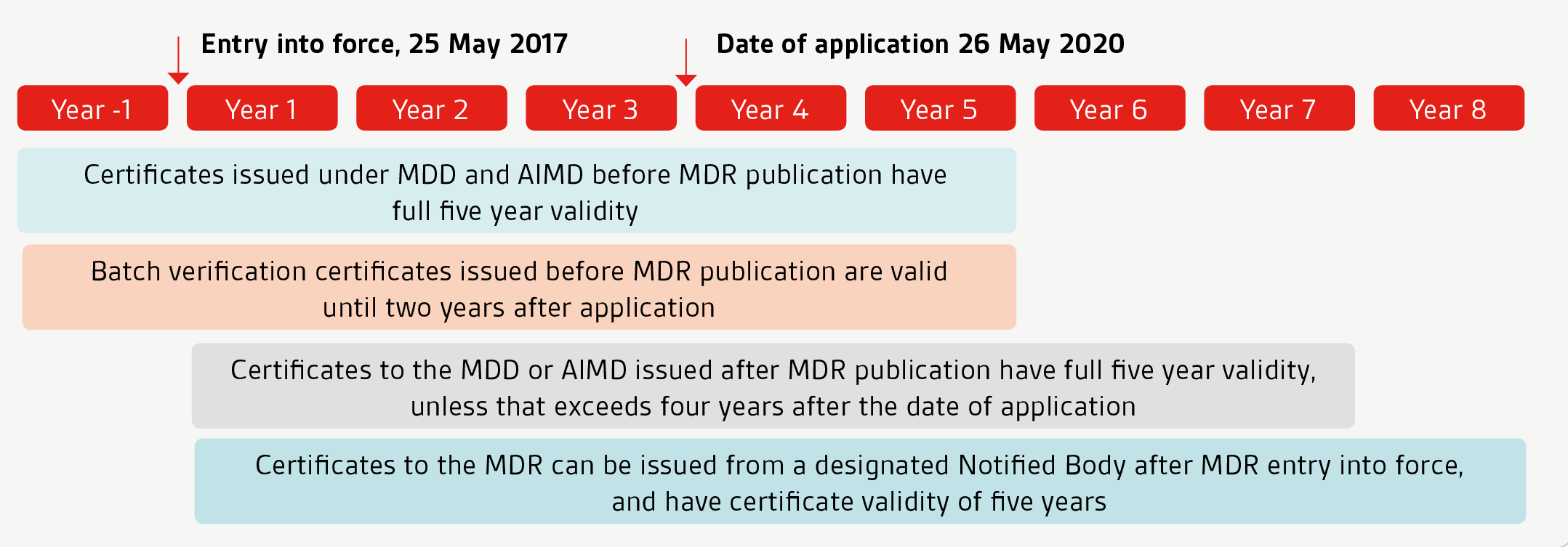
*Note: the blocks display the time period within which a certificate type can be valid, not the period of validity for a single certificate.
The new European Medical Devices Regulation was published in the Official Journal of the European Union on 5th May 2017. The Regulations will enter into force on May 25th 2017, marking the start of the transition period for manufacturers selling medical devices into Europe.
The MDR, replaces the Medical Devices Directive (93/42/EEC) and Active Implantable Medical Devices Directive (90/385/EEC), and has a transition period of three years. Manufacturers have the duration of the transition period to update their technical documentation and processes to meet the new requirements. Article 120 of the Regulation states a number of transitional provisions, and should be referred to for more detail.
No, the new requirements will be applied to all devices only when they are to be certified under MDR. After the transition period, devices not conforming to the MDR will need to be removed from the market.
BSI Group equips businesses with the necessary solutions to turn standards of best practice into habits of excellence. From assessment, certification and training to software solutions, advisory services and supply chain intelligence*, BSI provides the full solution to facilitate business improvement and helps clients drive performance, manage risk and grow sustainably.
For organizations large and small, BSI enables resilience by embedding excellence to create relevant, safer and more secure products/services leveraging the passion and expertise of our people. Renowned for its marks of excellence, BSI’s influence spans across multiple sectors with a particular focus on Aerospace, Automotive, Built Environment, Food, Retail, Healthcare and IT.
* If an organization is certified with BSI to a management system, please note that BSI will not provide consulting services for that particular management system due to impartiality requirements.
Contact us to learn more about training, certification and software tools.